Professor of Neurosurgery
Professor of Cell Biology
Professor in Molecular Genetics and Microbiology
Professor in Medicine
Member of the Duke Cancer Institute
Viral Cancer Immunotherapy
The Gromeier Laboratory is dedicated to unraveling molecular mechanisms of +strand RNA virus:host interaction that lend themselves to cancer immunotherapy.
Our research encompasses translation regulation, mitogenic signal transduction pathways and their role in protein synthesis control, innate antiviral immunity and clinical translational cancer immunotherapy.
Publications
View Gromeier Lab publications here.
Research Initiatives
Molecular Mechanisms of Protein Synthesis Regulation
A pivotal lever of gene regulation is control over ribosome recruitment to messenger RNAs. We are particularly interested in this process, as the outcome of infection with poliovirus (a +strand RNA virus whose genome functions as an mRNA) is defined by the ability of attracting (host) ribosomes to (viral) RNAs.
We are studying the mechanisms enabling eukaryotic translation initiation factors (eIFs) to recruit ribosomes and initiate protein synthesis at defined mRNAs, e.g. poliovirus genomes.
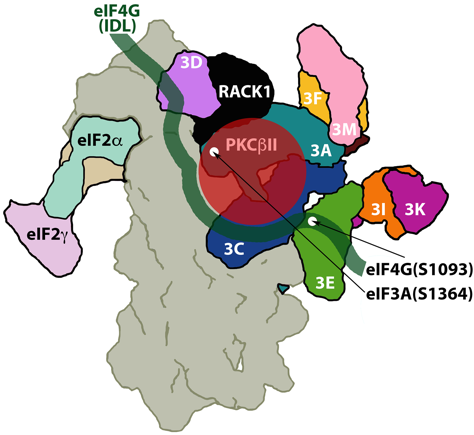
Fig 1: The small ribosomal subunit (gray; seen from the back), bound to eIF3 (9 of 13 subunits are shown in bright colors), eIF4G (green) and eIF2 (the a, b and g subunits are shown in pastel colors). The Receptor for Activated protein C Kinase (RACK1; black), associated with its high affinity ligand, protein kinase C bII (red).
Dobrikov MI, Dobrikova EY, Gromeier M. Ribosomal RACK1:PKCβII Phosphorylates eIF4G1(S1093) to Modulate Cap-Dependent and -Independent Translation Initiation. Mol Cell Biol (2018) PMID: 30012863.
Mitogenic Signals to Translation Machinery
Cells respond to extracellular cues by activating signal transduction networks that adjust protein synthesis rate to match the current conditions. The principal mitogenic signaling networks centered on Raf-ERK1/2 and PI3K/mTOR control cell growth and proliferation partly through converging signals on the translation apparatus.
We are unravelling the mechanisms of Raf-ERK1/2 and mTOR-mediated protein synthesis regulation in our quest to decipher virus:host relations in host (neoplastic) cells with unhinged mitogenic signal transduction networks or (antigen presenting) cells that are able to resist viral cytopathogenicity and experience sub-lethal infection.
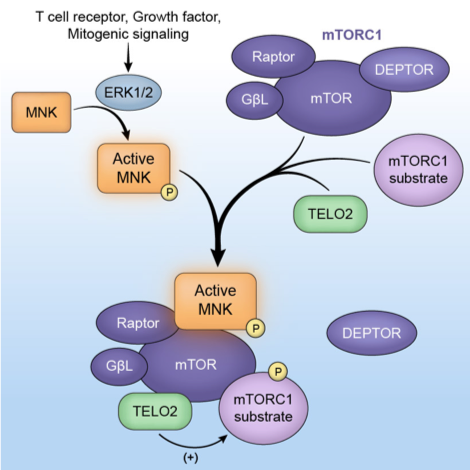
Fig 2: The downstream ERK1/2 substrate -MNK- interacts with mTOR and controls mTOR association with its substrates and with TELO2 in the mTORC1 complex. Active MNK modulates mTOR’s role in protein synthesis control.
Brown MC, Gromeier M. MNK Controls mTORC1-Substrate Association through Regulation of TELO2 Binding with mTORC1. Cell Rep (2017) PMID:28178522.
Mitogenic Signals to Translation Machinery
Cells respond to extracellular cues by activating signal transduction networks that adjust protein synthesis rate to match the current conditions. The principal mitogenic signaling networks centered on Raf-ERK1/2 and PI3K/mTOR control cell growth and proliferation partly through converging signals on the translation apparatus.
We are unravelling the mechanisms of Raf-ERK1/2 and mTOR-mediated protein synthesis regulation in our quest to decipher virus:host relations in host (neoplastic) cells with unhinged mitogenic signal transduction networks or (antigen presenting) cells that are able to resist viral cytopathogenicity and experience sub-lethal infection.

Fig 2: The downstream ERK1/2 substrate -MNK- interacts with mTOR and controls mTOR association with its substrates and with TELO2 in the mTORC1 complex. Active MNK modulates mTOR’s role in protein synthesis control.
Brown MC, Gromeier M. MNK Controls mTORC1-Substrate Association through Regulation of TELO2 Binding with mTORC1. Cell Rep (2017) PMID:28178522.
Oncolytic Virotherapy of Primary CNS Tumors
We are pursuing cancer immunotherapy with highly attenuated, recombinant poliovirus (PVSRIPO) in the clinic. Our basic mechanistic research (see above) informs a series of clinical initiatives in primary malignant brain tumors in adults and in children and in non-CNS tumors, e.g. triple-negative breast cancer and melanoma.
We are investigating the optimal use of PVSRIPO as an immunotherapy agent in the clinic, incl. combination with other immune-modulatory agents/standard of care, and the factors that govern the therapy response.
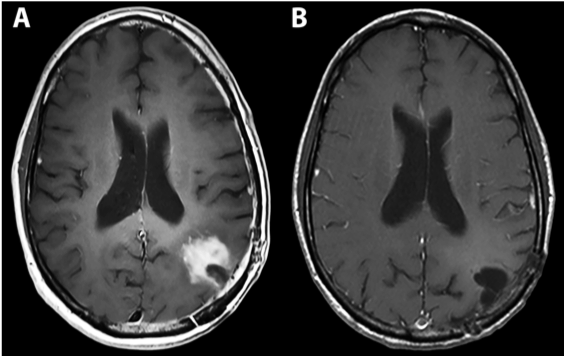
Fig 4: Complete response in a patient with recurrent GBM 15 months post PVSRIPO therapy.
Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, Bigner DD. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N Engl J Med (2018) PMID: 29943666.
Personnel
Lab Director

Matthias Gromeier, MD
Professor of Neurosurgery
Duke University Medical Center
Box 3020
Durham, NC 27710
Phone: 919-668-6205
Fax: 919-681-4991
E-mail: grome001@mc.duke.edu
Postdoctoral Fellows
- Elena Y. Dobrikova, PhD
- Mikhail I. Dobrikov, PhD
- Michael C. Brown, PhD
- Jeffrey D. Bryant, PhD
PhD Students
- Ross W. Walton
- Mubeen Mosaheb
- Jonathan P. Kastan
- Yuanfan Yang, MD
Research Tech
- Zachary P. McKay