Report: Epilepsy
Deep Brain Stimulation for Epilepsy
Since performing the first deep brain stimulation (DBS) procedure for epilepsy in North Carolina in 2019, neurosurgeon Derek Southwell, MD, PhD, has treated 35 patients.
After DBS, most patients will experience a great improvement in quality of life, with an average reduction in seizure frequency of approximately 70%.
“DBS can be applied in cases where it’s difficult to fully identify where seizures originate,” says Southwell, the surgical director of the Duke Comprehensive Epilepsy Center. “It is also a valuable option in cases where the seizure onset areas are known, but they happen to be multiple, or too large, or too vital for function to remove.”
Next-Level Brain Imaging Uses Holograms to Aid Electrode Placement in Epileptic Brains

McIntyre and colleagues are creating next-level MRI technology that gets neurosurgeons the information they need to place electrodes in epileptic brains. “Electrode placement is about finding the magic spot for that individual’s brain,” says McIntyre. “It’s about the best place to stimulate a person’s brain based on their type of epilepsy and their unique health.”
Being able to find that magic spot is why Duke is a world-wide leader for deep-brain stimulation targeting, electrode placement, and programming — Duke is thinking beyond this dimension.
The Duke team has created human head models with holographic imaging that allow surgeons to see the anatomy of patient-specific brains in 3D. Not only that, as a neurosurgical team collaborates to plan a patient’s unique treatment, they can do so remotely while interacting in the hologram.
“It’s an amazing application,” McIntrye says. “We’ve got good models that have been validated. Now, it’s time to put them into clinical testing.”
Cameron McIntyre, PhD, is a bioengineer and Duke Neurosurgery faculty member who is advancing Duke’s work in deep brain stimulation for epilepsy. “Epilepsy is different for every person who suffers from it, even if they have the same type of epilepsy,” says McIntyre. “But they all have the same hope: prevent a seizure before it starts.”
Interneuron Transplantation As a New Therapy for Epilepsy
Neurosurgeon Derek Southwell, MD, PhD, is exploring how the transplantation of interneurons — brain cells that provide inhibitory signals to partner brain cells — can be made safe and effective as a therapy for epilepsy.
Southwell and others have shown that interneurons can be transplanted into the rodent brain, where they modify the neural circuits of the recipient. Grafting new inhibitory interneurons into the recipient brain can reduce the activity patterns that give rise to seizures. If successful in humans, this therapeutic approach has the potential to reduce epileptic seizures with less surgical invasiveness, risk, and impact on brain function.
This technology has potential implications beyond epilepsy, says Allan Friedman, MD, Duke neurosurgeon and past chair. “Dr. Southwell’s work has so much potential to restore function to patients who have lost function secondary to stroke, trauma, or neurological diseases,” he adds.
Shedding Light on Human Neural Circuitry and Advancing Epilepsy Research
Experimental approaches for performing live, cellular studies of the human brain are rare. Instead, scientists have used lab models such as mice to study how cell types organize into neural circuits, the basic functional elements of the nervous system. As a result, we know far less about human brain cells, and how they function in neural circuits.
Derek Southwell, MD, PhD, assistant professor of neurosurgery and surgical director of the Duke Comprehensive Epilepsy Center, has collaborated with Duke professor of neurobiology, Joshua Huang, PhD, to advance strategies for studying human neural circuits.Using human brain tissues collected from neurosurgical patients and CellREADR, an RNA sensor
method for cell type-specific access developed by Huang and Yongjun Qian, PhD, the Southwell Lab is now exploring how different types of neurons function in live human brain tissues.
Their findings were published in the journal Nature, October 5, 2022.
By characterizing human cell populations and investigating how they function in both healthy and diseased human neural circuits, the Southwell lab is exploring the cellular and circuit underpinnings of conditions such as epilepsy. This work is shaping the lab's ongoing efforts to advance cell and gene therapy strategies for neural circuit repair.
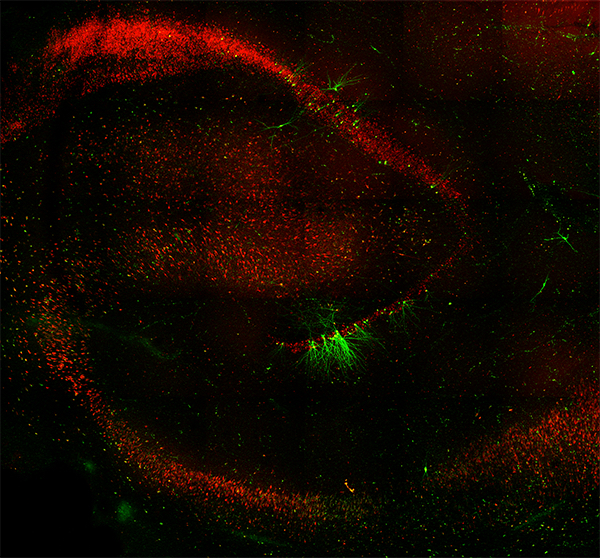
fluorescent protein), enabled live studies of a specific class of inhibitory interneuron in the epileptic brain tissue.
All Chapters
Home / Brain Tumor / Epilepsy / Cerebrovascular & Skull Base / Pediatric / Chronic Pain / Trauma / Movement Disorders / Brain & Spine Metastasis / Spine / Global / Residency / Top Papers
