Report: Brain Tumor
Established in 1937, The Preston Robert Tisch Brain Tumor Center (PRTBTC) was one of the first brain tumor research and clinical programs in the United States, and it is part of one of the original Comprehensive Cancer Centers, the National Cancer Institute’s highest mark of excellence.
-
The PRTBTC sees an average of 900 new adult brain tumor patients and 80 new pediatric brain tumor patients annually, from the local area and around the world.
-
Three PRTBTC faculty members (Darell Bigner, MD, PhD, Henry Friedman, MD, and Roger McLendon, MD) are among the top 20 most-cited authors worldwide for glioblastoma.
-
Twenty-eight members of the Center hold 25 National Institutes of Health grants.
-
In March 2023, Duke hosted the International Conference on Brain Tumor Research and Therapy. Over 130 of the world's top brain tumor researchers traveled from Japan, Australia, Norway, Korea,Germany, Spain, the Netherlands, Canada, the UK and across the U.S.
-
In May 2023, David Ashley, MBBS, PhD, director of the Brain Tumor Center, and Henry Friedman, MD, participated in the White House Cancer Moonshot Forum on Brain Cancers
The Brain Tumor Omics Program
“The Brain Tumor Omics Program will do Duke doing what Duke does best – taking revolutionary ideas and translating them into therapeutics,” said Patel.
A first-of-its-kind research program, the Duke Brain Tumor Omics Program (BTOP), is focused on the interrelated omics fields (genomics, transcriptomics, epigenetics, proteomics, and metabolomics) to better understand brain tumor development and progression.
The time for such a program has come, says neurosurgeon- scientist Anoop Patel, MD. “The technology for genetic sequencing has improved dramatically over the decades,” he said. “We’ve been doing genetic sequencing for other cancers for a long time, and some have turned into chronic diseases that can be managed. Brain tumors haven’t seen that.”
The program is a huge undertaking, and technology is one of its cornerstones. “We have the most cutting-edge technology available to our researchers to conduct multi-omic approaches, looking at proteins, DNA, metabolism, all the ways to characterize cancer cells,” said Patel.
Another cornerstone is patient-specific profiling. “Brain tumors contain a mixture of different cells that drive tumor development or influence therapy response,” said Simon Gregory, PhD, director of the BTOP and faculty member in the Duke Department of Neurosurgery. “By breaking the tumor down to its component cells and then independently assembling them, we have a much clearer idea of the pathways associated with cancer progression and response.”
To profile brain tumors, investigators are using data from traditional bulk approaches and employing emerging technologies such as single cell and spatial transcriptome profiling. These new technologies – some of it not available anywhere else in the world – present an opportunity to understand the microenvironment of brain tumors at unprecedented resolution.
Finally, the program is positioned to take advantage of the best Duke has to offer: interdisciplinary partnerships not only within the Brain Tumor Center but across the university, and Duke’s unique prowess in translational research.

A New Approach to Meningioma Takes Inspiration From Another Program's Success
Duke is building a most valuable program: Meningioma, vestibular schwannoma, pituitary tumors (MVP). The program is taking a page from the Duke Center for Brain and Spine Metastasis, one of the fastest growing clinical programs at Duke (see related story on page 23). Like the metastasis program, the MVP model is research-forward and focused on clinical trial activation.
Also like the metastasis program, the MVP will draw on multidisciplinary leadership in neurosurgery, neuro-oncology, radiation oncology, radiology, basic science, and translational genomic research. Peter Fecci, MD, PhD, director of the Duke Center for Brain and Spine Metastasis; Kyle Walsh, PhD, director the Division of Neuro-Epidemiology; and Gerald Grant, MD, chair of the Department of
Neurosurgery, are providing leadership to the new program. “These tumors are both more common and more treatable than gliomas,” said Walsh, “but about 10% of these tumors behave very aggressively and have 5-year survival rates of just 50%. We currently lack robust classifiers that can accurately predict which cases fall into that 10% and merit more aggressive treatment strategies.”
Genomics plays a key role in the MVP’s research, with whole-exome and whole-transcriptome profiling performed on all resected tumors to guide clinical decision-making and to inform a new generation of molecularly-informed therapeutic trial designs.
Defeating Brain Tumors Before They Start

The lab of David Ashley, MBBS, PhD, is studying the STING protein, which signals the immune system to repair DNA damage. They found that this pathway is turned off in adults with brain tumors as well as during normal fetal development.
They wondered if brain tumors take advantage of other mechanisms that are meant to operate only before a baby is born.
With genomic technologies, in collaboration with partners in Norway, they’re looking for the “cell of origin” for brain tumors. “Certain genes switch on and off as cells in the brain mature,” said Ashley, who is director of the Brain Tumor Center. “But there's a type of cell that's meant to disappear and not exist in the adult brain. When we look at that cell, it looks very much like a glioblastoma.”
By studying human fetal atlases and brain tumor tissue, the team found evidence that this cell is the “seed cell" that initiates glioblastoma. “This implies that the earliest steps in forming a glioblastoma occur in the developing fetus,” Ashley said.
If confirmed, these findings could lead to a blood test to detect this seed cell in babies, indicating risk for a brain tumor later in life.
“We think the key is that the fetal cells are persisting in adults,” Ashley said. “And if we could detect these cells in people at risk, it's conceivable that we could prevent brain tumors by developing medications to prevent the cells of origin from progressing.”
The Botha Chan Low-Grade Glioma Research Consortium
Research scientists from the Preston Robert Tisch Brain Tumor Center at Duke and the Pacific Pediatric Neuro-Oncology Consortium are joining together to create a research environment specific to low-grade gliomas in children and adults.
The goal of the collaborative, according to Duke leaders Gerald Grant, MD, and David Ashley, MBBS, PhD, is to deliver novel insights that will lead to state-of-the-art clinical trials. Duke Neurosurgery is grateful to the Botha-Chan family who provided the seed funding to make this consortium possible.
The program’s research efforts will be led by the outstanding investigators from these academic centers and consortia focused on low grade gliomas.
- Duke University
- University of California San Francisco
- Stanford University
- Dana-Farber Cancer Institute
- Brigham and Women’s Hospital
- Children’s Hospital of Philadelphia
- Northwestern University Lurie Children’s Hospital
- University Children’s Hospital, Zurich
- German Cancer Research Institute
- Pacific Pediatric Neuro-Oncology Consortium
- Children’s Brain Tumor Tissue Consortium
New Approaches to Oligodendroglioma
Treatments for oligodendrogliomas — invasive tumors that eventually progress to high grade — have not changed over the last 10 years. New treatments are needed, but also new markers to identify the tumors that will be more aggressive.
Duke Neurosurgery research scientist Giselle López, MD, PhD, is working to solve these problems by:
Assessing histologic and molecular biomarkers predictive of progression-free survival and overall survival in oligodendroglioma.
“Identifying these markers can help predict which tumors will behave aggressively and which ones will grow more slowly, ensuring patients receive the correct treatment at the correct time,” said López. “This enables us to identify patients who would benefit from early aggressive treatment.” And for other patients, knowing that their tumor is growing slowly may mean they can delay chemotherapy and radiation and have another day without the complications of these treatments.
Identifying new therapeutic targets using multi-omics approaches.
These approaches include spatial transcriptomics, single nucleus RNA sequencing, and other modalities to identify pathways associated with increased proliferation and stemness. Lopéz and team are bringing new oligodendroglioma cell lines in house to rapidly validate these findings in an in vitro model system.

Bringing Top-Notch Brain Tumor Care to Veterans
Give all of our veterans access to the very best neuro-oncology care, no matter where they live.
That’s what Duke neuro-oncologist Maggie Johnson, MD, and colleagues are doing, using a two-pronged approach.
First, Johnson and neuro-oncologists around the country are working with the US Department of Veterans Affairs (VA)’s National Oncology Program to give veterans top-notch brain tumor care using virtual-health technologies. The convenience the technology provides, especially for veterans in rural areas, cannot be understated. The VA calls it their National TeleOncology Service, or NTO for short.
For veterans, it means they can be surrounded by their loved ones at home while still receiving state-of-the-art care. It also means not having to travel to appointments.
“So far, we have teamed up with 44 different VA sites,” said Johnson. “To make sure veterans receive
the care they deserve and care that’s on par with institutions like Duke, the VA is increasing the volume of patients we see, the number of sites we serve, and virtual care opportunities.
"In addition, Johnson spearheaded the creation of the VA’s first National Virtual Brain Tumor Board, and serves as its chair. “The virtual tumor boards mean that any provider at any VA anywhere can present abrain tumor case they need help with,” she said. “I present my difficult VA cases just like I would a Duke tumor board.”
The virtual tumor board includes specialists in neuro-oncology, neurosurgery, radiation oncology, and neuro-pathology who all work for the VA in some capacity.
Opening the Blood-Brain Barrier
A central challenge in the treatment of glioblastoma lies in the limited delivery of therapeutics to the tumor itself, due in part to the constraints of the blood-brain barrier. In the lab, Gerald Grant, MD, and team are working to better understand how to open the blood-brain barrier, selectively and reversibly, to deliver promising targeted agents to brain tumors.
Grant’s work focuses on in vivo multi-photon microscopy imaging, which provides cellular resolution to study changes in a temporal and quantitative fashion. A cranial window model is coupled with a 2-photon microscope to closely track the tumor growing over time in an orthotopic tumor model in mice.
“Through our mouse work, we are able to visualize fluorescent tracers cross the blood-brain barrier into tumors in real time,” said Grant. “We can apply these same techniques in the operating room to precisely identify the tumor margin and to deliver catheters to the tumor bed through convection-enhanced delivery.”
Mechanism Identified For Drug Resistance in Glioblastoma
Duke Neurosurgery researchers made a break-through in understanding why glioblastoma can be impervious to immunotherapies that have been highly effective in other cancers.
The findings of researchers in the lab of John Sampson, MD, PhD, were published in Nature Communications in October 2022. “Our approach in this work was to find environmental factors that could make glioblastoma more vulnerable to immunotherapies,” said lead author William H. Tomaszewski, PhD.
The Duke team focused on the molecule CaMKK2, which is present in neurons and immune cells. In mouse studies, T-cells were inhibited from attacking brain tumor cells in animals with high levels of CaMKK2. Among mice bred without CaMKK2, T-cells remained aggressive cancer killers.
CaMMK2 also changed the behavior of macrophages, making these immune cells less helpful to T-cells. When CaMMK2 was eliminated, the macrophages aided in tumor killing.
The findings suggest that CaMKK2 represents a potential therapeutic target. If CaMMK2 could be inhibited, we might be able to unleash the power of the whole class of immunotherapy drugs.
Supporting Quality of Life
When caring for brain tumor patients, supporting a better quality of life can be as important as treating the cancer. “It’s about creating better days for all impacted by brain cancer,” says neuro-oncologist Katy Peters, MD, PhD.
To this end, Peters and her team are designing clinical protocols that seek to answer questions such as:
- What medications are best to prevent nausea and vomiting during chemotherapy?
- Can exercise improve my thinking? My survival?
- How does paying for expensive medications affect my quality of life?
“For example, a current trial involves using novel technology to alleviate the anxiety associated with undergoing MRI scans, a common procedure required for most patients with brain tumors,” said Peters.
In 2022, Peters and colleagues launched a virtual symposium to present our research and highlight our collaborations in supportive care around the world.
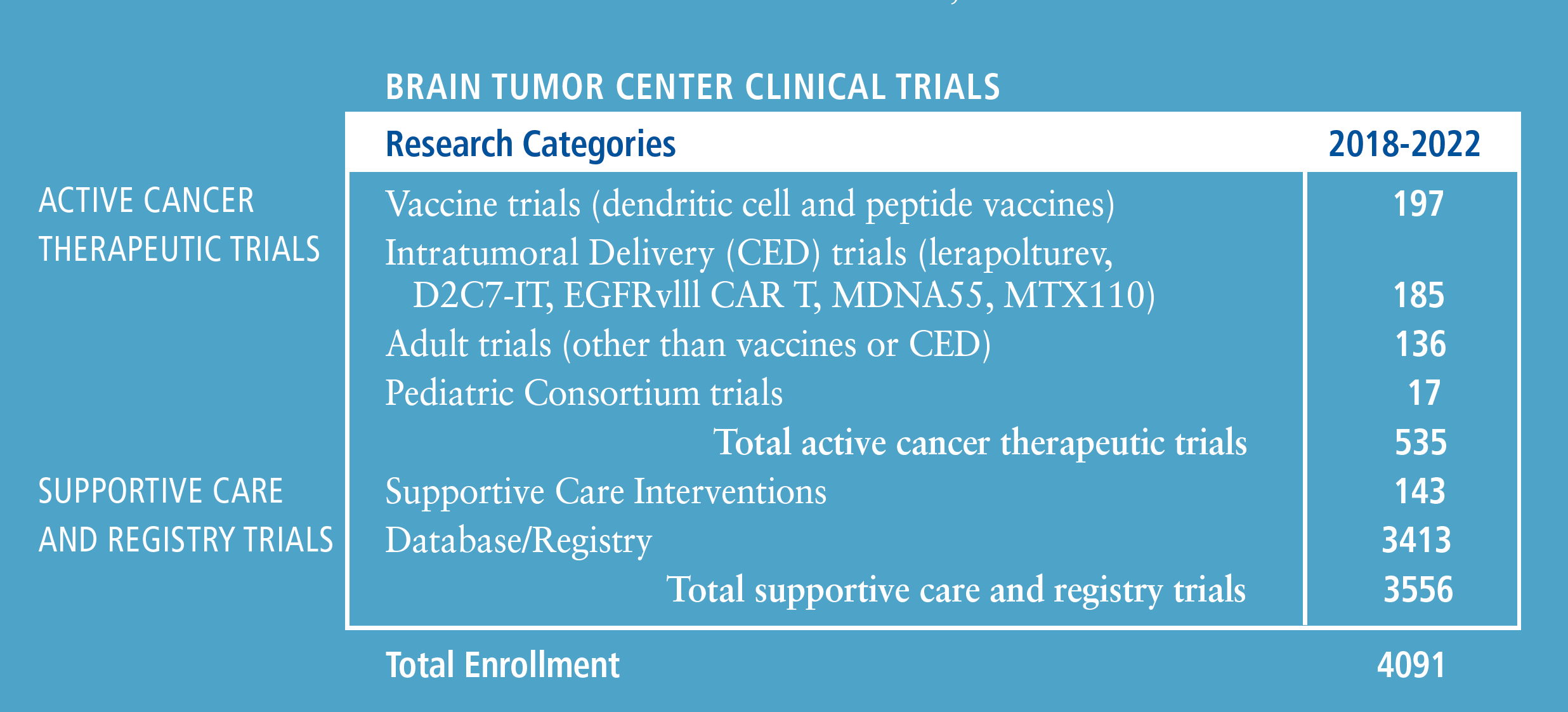
Clinical Trial Capabilities in the Brain Tumor Center
The Preston Robert Tisch Brain Tumor Center (PRTBTC) is leading the charge to transform patient outcomes through the development of new treatment standards.
Through unparalleled collaboration between scientists and clinicians, Duke is inventing and translating novel therapeutics into clinical trials that incorporate trial designs and techniques.
Annick Desjardins, MD, PhD, professor of neurosurgery and neurology, is director of clinical research.
DUKE BTC INVOLVEMENT
IN FDA APPROVAL
• Carmustine wafers
• Temozolomide
• Bevacizumab
LICENSING PARTNERS
Translation of locally discovered lab innovations to multicenter clinical trials
• CDX110 (Rindopepimut), Celldex
• Lerapolturev (PVSRIPO), Istari Oncology
• DC Vaccines pp65, Immunomix
• BMX-001, Biomimetix
• Anti-Tenascin, Bradmer
All Chapters
Home / Brain Tumor / Epilepsy / Cerebrovascular & Skull Base / Pediatric / Chronic Pain / Trauma / Movement Disorders / Brain & Spine Metastasis / Spine / Global / Residency / Top Papers
